Cefoperazone
- FOB Price: USD: 0.10-0.10 /Gram Get Latest Price
- Min.Order: 1 Gram
- Payment Terms: L/C,D/A,D/P,T/T,
- Available Specifications:
EP USP JP CP IP(0.1-0.2)GramEP USP JP CP IP(0.3-0.4)GramTechnical Grade(0.5-0.6)Gram
- Product Details
Keywords
- Cefoperazone Sodium
- Cefoperazone
- Sodium Cefoperazone
Quick Details
- ProName: Cefoperazone
- Appearance: White to off white powder/cake
- Application: Treatment of invasive aspergillosis in...
- DeliveryTime: 7-10 Days
- PackAge: 1 kgs/Tin, 25kgs/Tin
- Port: Any port of China
- ProductionCapacity: 1 Metric Ton/Month
- Purity: 99.9%
- Storage: 0-6℃ storage
- Transportation: By Air, By Sea or By Courier
- LimitNum: 1 Gram
- Related Substances: 0.1%Max
- Residue on Ignition: 0.3%Max
- Heavy Metal: 5 PPM Max.
- Valid Period: 36 Months
- Appearance: White to off white powder/cake
- Protein binding: 97%
- Metabolism: Slowly metabolized by hydrolysis and N...
- CAS: 54651-05-7
- Formula: C52H81N7O16
- Molecular mass: 1074.27
- Routes of Absorption: Inhalation,ingestion,eye and skin abso...
- Process: Caspofungin is semisynthesized from pn...
- Products of Degradation:: The product itself and its products of...
- General Use: Antifungal
- Stability: Stable
Superiority
|
Echinocandin B |
|
|
Verified By |
GMP,FDA,CEP,COS,ANDA |
|
Service |
1, Professional Techincal Guidence
4, Lifetime customer service since first deal |
|
Process |
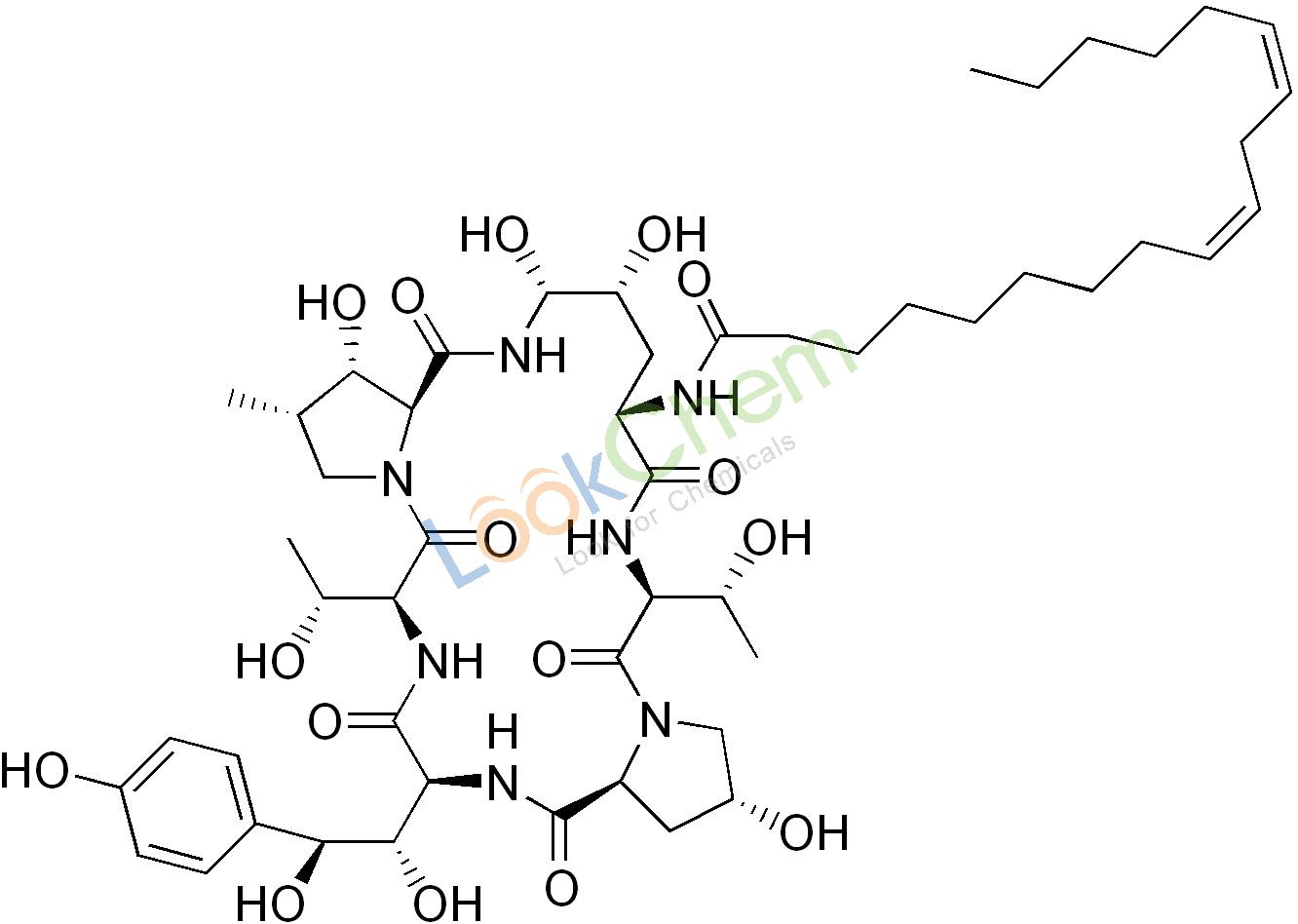
Echinocandin B, a lipopeptide, is a naturally occurring cyclic hexapeptide with a linoleoyl side chain. It belongs to a class of antifungal agents called echinocandins, which inhibits the synthesis of glucan, a major component of the fungal cell wall, via noncompetitive inhibition of a crucial enzyme, β-(1,3)-D-glucan synthase. Echinocandin B is a fermentation product of Aspergillus nidulans and the closely related species, A. rugulosus; discovered in 1974 in A. nidulans var. echinulatus strain A 32204 in Germany, it was the first of the echinocandin class of antifungals. Echninocandin B can undergo deacylation (removal of the lipid side chain) by the action of a deacylase enzyme from the filamentous bacterium Actinoplanes utahensis, which catalyzes the cleavage of the linoleoyl side chain; in three subsequent synthetic steps, including a chemical reacylation, the antifungal drug anidulafungin is synthesized.
|
Details
|
Echinocandin B |
|
Echinocandin B, Echinocandin B, a lipopeptide, is a naturally occurring cyclic hexapeptide with a linoleoyl side chain. It belongs to a class of antifungal agents called echinocandins, which inhibits the synthesis of glucan, a major component of the fungal cell wall, via noncompetitive inhibition of a crucial enzyme, β-(1,3)-D-glucan synthase. Echinocandin B is a fermentation product of Aspergillus nidulans and the closely related species, A. rugulosus; discovered in 1974 in A. nidulans var. echinulatus strain A 32204 in Germany, it was the first of the echinocandin class of antifungals. Echninocandin B can undergo deacylation (removal of the lipid side chain) by the action of a deacylase enzyme from the filamentous bacterium Actinoplanes utahensis, which catalyzes the cleavage of the linoleoyl side chain; in three subsequent synthetic steps, including a chemical reacylation, the antifungal drug anidulafungin is synthesized. yield of echinocandin B nucleus is low. In our study, the echinocandin B deacylase gene was systematically overexpressed by genetic engineering in its original producer, A. utahensis, and in the heterologous hosts Streptomyces lividans TK24 andStreptomyces albus. The introduction of additional copies of the gene, under the control of PermE* or its native promoter, into hosts showed significant increases in its transcription level and in the efficiency of the bioconversion of echinocandin B to its nucleus. The conditions for the cultivation and bioconversion of A. utahensis have been optimized further to improve production. As a result, while the wild-type strain initially produced 0.36 g/liter, a concentration of 4.21 g/liter was obtained after the generation of a strain with additional copies of the gene and further optimization of the reaction conditions. These results are useful for enhancing echinocandin B nucleus production in A. utahensis. Our study could enable the engineering of commercially useful echinocandin B nucleus-overproducing stains. Fungal infections are being seen in ever-increasing numbers, largely because of the increase in the size of the population at risk over the past 20 years.
This population includes cancer patients, transplant recipients, and other individuals receiving immunosuppressive treatment. These people are at greater risk than others owing to their weakened immune systems and the chronic nature of diseases ( 1,–,3). The increased incidence of invasive fungal infections has created a major challenge for health care professionals. Since cell walls are present in fungal cells but absent in animal cells, the fungal cell wall perhaps represents the ideal target for the therapeutic treatment of fungal pathogens in humans (4, 5). The development of echinocandins, the first class of antifungals to target the fungal cell wall, was a milestone in antifungal chemotherapy (6, 7). Three semisynthetic echinocandin derivatives have been developed for clinical use: caspofungin, micafungin, and anidulafungin (8). Their strengths include low toxicity, rapid fungicidal activity against most isolates of Candida spp., and predictable, favorable kinetics allowing once-a-day dosing. Besides Candida spp., their inhibitory spectrum includes Aspergillus spp. and Pneumocystis jirovecii, but not Cryptococcus neoformans (9, 10). Echinocandin B (ECB), obtained by the fermentation of Aspergillus nidulans andAspergillus rugulosus, is known as one of the natural cyclic hexapeptides that have a linoleoyl side chain, which inhibits a crucial enzyme in fungal cell wall biosynthesis, β-(1,3)-D-glucan synthase (11). ECB can be modified by enzymatic deacylation to a cyclic hexapeptide without a linoleoyl side chain and by subsequent chemical reacylation to generate a few therapeutic antifungal agents for clinical practice, such as anidulafungin (12,–,15). A deacylase fromActinoplanes utahensis NRRL 12052 catalyzes the cleavage of the linoleoyl side chain from ECB (Fig. 1), an essential reaction for the three subsequent synthetic steps (16). The enzyme is a membrane-associated heterodimer composed of 63-kDa and 18- to-20-kDa subunits, and the expression of its activity is not affected by any cofactors, metal ion chelators, or reducing agents. In addition to that of ECB, this deacylase mediates the cleavage of aculeacin A, FR901379, various semisynthetic ECB derivatives, daptomycin and its three derivatives, teicoplanin, pseudomycin A, and capsaicins (17, 18). Thus, it may become increasingly significant as a pharmaceutical biocatalyst.
|
|
|
|
Packaging |
|
The packaging can be customized, normally, 1kgs/Tin, or 5kgs/Tin or per drums |
|
|
|
Company Introduction |
|
Top Pharm Chemical Group is specialized in the New Drug Researching and Developing, and mainly manufacture Chemical Intermediates, Pharmaceutical APIs and Pilot Production. To meet customers regulatory needs, we can offer products from gram to kilo and tons scale, and we can also support our customers with the DMF and other documents to Complete the registration. Our Targets: "Best Technic; Better Quality; Better Price; Better Service" Main Products: Anidulafungin,Micafungin,Caspofungin,Posaconazole etc. (Anti-Infective) Ixabepilone, Argatroban, Decitabine, Everolimus etc. (Anti-Tumor) Epothilone B, Tacrolimus, Rapamycin,Temsirolimus etc. (Fermentation) Bimatoprost, Latanoprost, Travoprost, Cloprostenol etc.(Prostaglandins) |
|
|
|
|
|
|
|
|
|
|